Bone Adhesive
-
Innovation lab 2026
PBC Biomed hosted its first Innovation Lab of 2026 in Leeds last week, graciously hosted by Professor Peter Giannoudis. We were delighted to be joined by an outstanding international faculty: – Dr Kostas Triantafillou (UT Medical, Knoxville)– Professor Thierry Bégué (Antoine Béclère Hospital)– Professor Taco Blokhuis (Maastricht University Medical Center)– Dr Thomas A. (Toney) Russell
-

A great week put down at Orthopaedic Trauma Association
What a fantastic week at the 2025 Orthopaedic Trauma Association (OTA) meeting in Phoenix! The PBC Biomed booth attracted huge interest from clinicians and industry partners alike. Our team showcased OsStic™ bioadhesive, ReFeel™ Nerve Repair Solution, and our ARC and VAPs technologies through countless live demonstrations — sparking many great conversations about the future of
-
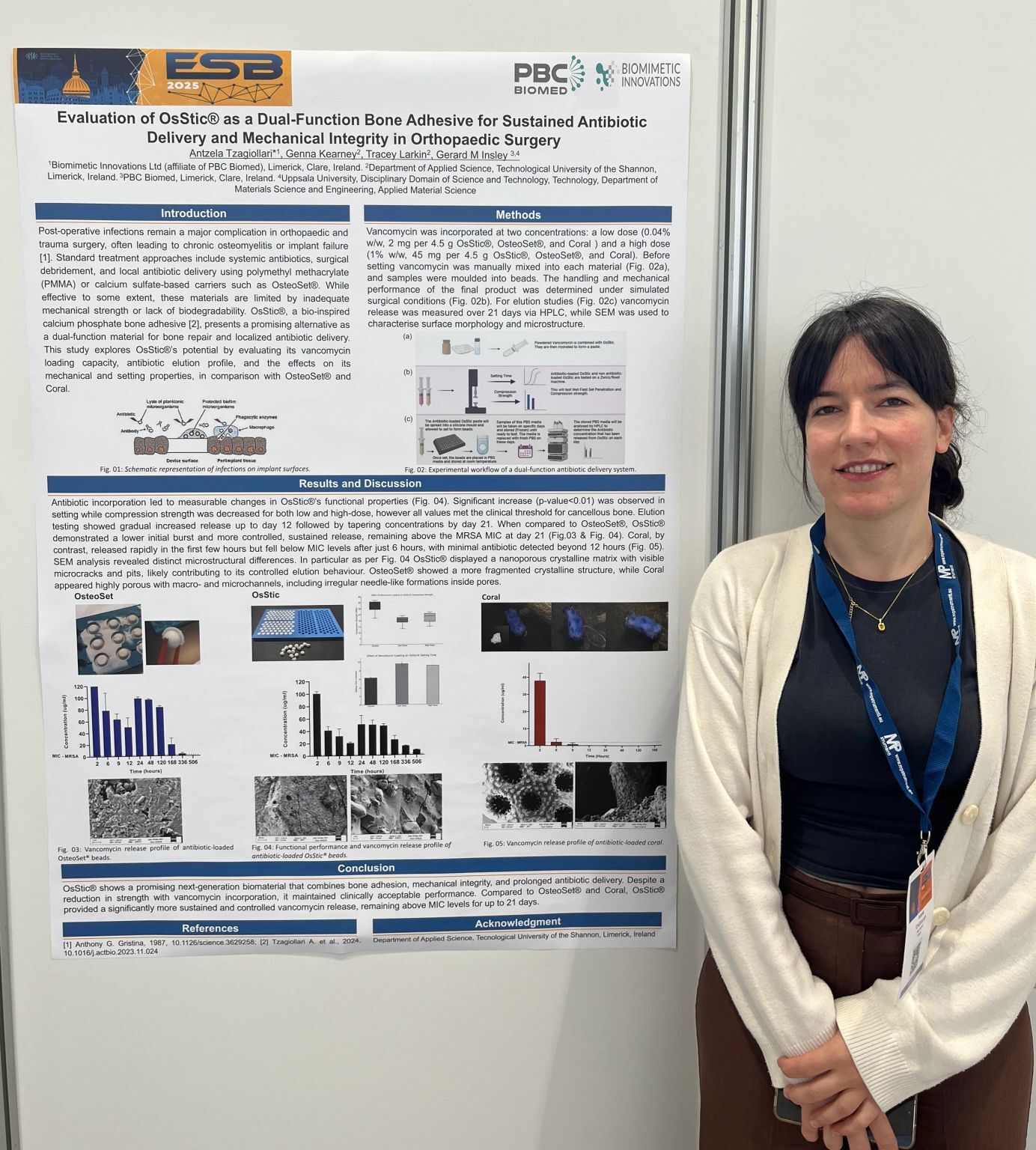
PBC Biomed Poster Presentation at ESB 2025
Congratulations to Senior R&D Engineer Antzela Tzagiollari on her poster presentation at the ESB 2025 event yesterday, highlighting her team’s research on OsStic® as a promising next-generation biomaterial. Their findings show that OsStic® uniquely combines bone adhesion, mechanical strength, and prolonged antibiotic delivery. While incorporating vancomycin reduced its strength, OsStic® still demonstrated clinically acceptable performance.
-

First Call for Papers – Bioadhesive Symposium at ESB 2026
We are excited to announce our first call for papers for the PBC Biomed #Bioadhesive Symposium, which will be held as part of next year’s ESB Meeting in Antwerp, Belgium. The symposium will be chaired by Prof. Nicholas Dunne (DCU, Ireland) and feature keynote lectures from Prof. Håkan Engqvist (Uppsala University, Sweden), Dr. Marc Bohner
-

PBC Biomed are attending ESB 2025 in Turin
PBC Biomed is excited to be attending this year’s European Society for Biomaterials (ESB) meeting in Turin! Our Senior R&D Engineer, Antzela Tzagiollari, will be presenting her poster titled: “Evaluation of OsStic® as a Dual-Function Bone Adhesive for Sustained Antibiotic Delivery and Mechanical Integrity in Orthopaedic Surgery.” We’re also pleased to announce that we are
-

PBC BioVet Team showcase OssiVet® in the US!
We’re hitting the road!The PBC BioVet team on are tour across the state of Texas in the US to showcase OssiVet®, our advanced veterinary bone adhesive making waves in animal orthopedic surgery.From Dallas to Houston and Austin, in-person demos, clinical discussions, and hands-on presentations on how OssiVet® can:– Support fracture management– Improve implant fixation Remodel into new bone
-

PBC BioVet at ECVS Antwerp
Thank you to everyone who stopped by the PBC BioVet booth at ECVS 2025 in Antwerp, Belgium!It was a pleasure meeting so many veterinary professionals, exchanging clinical insights, and showcasing how OssiVet® is helping shape the future of orthopedic surgery.The event was a great success, and we truly appreciate your interest, questions, and engagementFor those
-

We’re heading to the ECVS Congress in Antwerp!
We’re heading to the ECVS Congress in Antwerp! PBC BioVet is proud to participate in the European College of Veterinary Surgeons (ECVS) Congress from July 3–5 in Antwerp, Belgium. 🧬 Visit us at Booth #20 to explore how OssiVet®, our advanced bone adhesive, is transforming orthopedic surgery in veterinary practice. 💬 Learn how this innovative
-

The PBC Biomed Team are delighted to celebrate the success of another exceptional #ESTROT event following our time in Leeds last week.
The PBC Biomed Team are delighted to celebrate the success of another exceptional #ESTROT event following our time in Leeds last week. Our ‘Strengthening The Bond’ workshop on our #breakthrough bio-adhesive, OsStic®, attracted great interest and discussion, featuring a futuristic look at Bone Healing led by Prof. Thomas A. (Toney) Russell MD, including an interactive
-

Join Us for the OssiVet® Webinar – April 14, 2025!
📢 Join Us for the OssiVet® Webinar – April 14, 2025! 🦴💻 We’re excited to invite veterinary professionals to our upcoming OssiVet® Educational Webinar on Monday, April 14th, 2025. Discover how OssiVet®, our advanced orthopedic bone bioadhesive, is transforming fracture management and joint stabilization in veterinary surgery. From science to real clinical cases, we’ll cover
Search News Items
Categories
- Awards (11)
- Biomaterials (24)
- Biovet (13)
- Bone Adhesive (20)
- Bone Disease Solution (4)
- Bone Healing (14)
- Certification (1)
- Client (2)
- Collaboration (30)
- Conferences (24)
- Contract Manufacturing (3)
- Day One Trauma Charity (4)
- Development (21)
- Events (65)
- Government Funding (3)
- Lectures (7)
- Medical Devices (9)
- Medical Implants (6)
- Memphis Office (8)
- New Hire (11)
- On TV/Radio (2)
- Partnership (6)
- Press Release (8)
- Publications (7)
- ReFeelNerveRepairSolution (12)
- Regenerative Medicine (15)
- Relationship-building (18)
- Research (18)
- Sports & Social (3)
- Sports Partners (9)
- Student Placements (3)
- Uncategorized (6)
- Upcoming Events (6)
- US Office (6)

